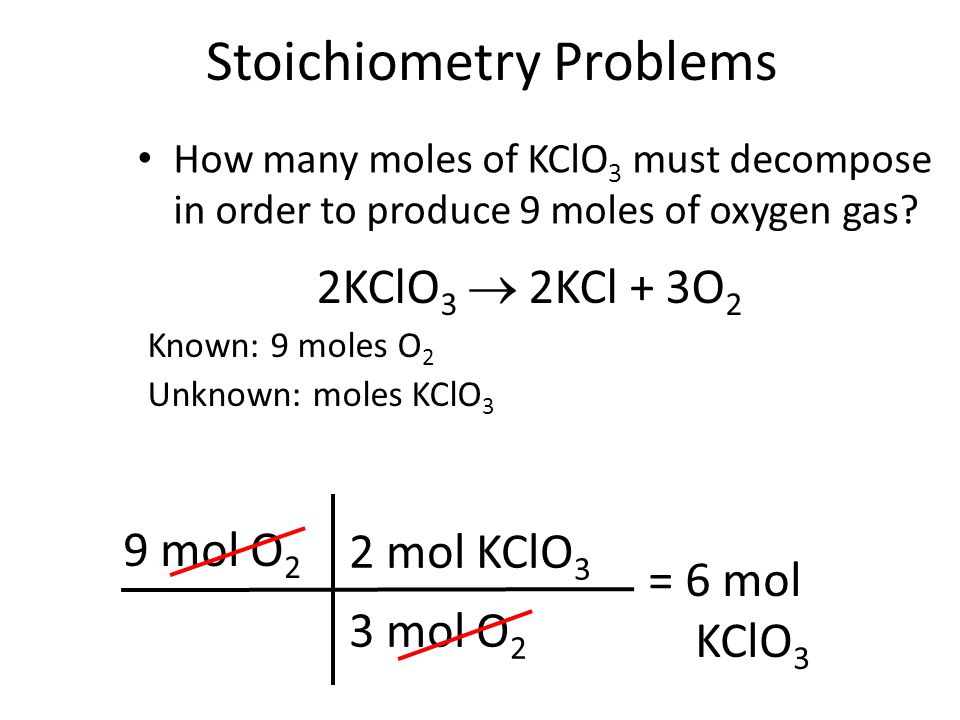
Step 1. Write the balanced equation. 2KClO3 → 2KCl+ 3O2 Step 2. Calculate the moles of KClO3. 10.0g KClO₃ × 1 mol KClO3 122.6g KClO₃ = 0.08156 mol KClO3 Step 3. Calculate the moles of O2. The balanced equation tells us that 2 mol KClO3 give 3 mol O2. Therefore, 0.08156mol KClO₃ × 3 mol O2 2mol KClO₃ = 0.1223 mol O2 Step 4.
Converting Mass of Reactant to Mass of Product — Examples – Expii
1 Answer: 0.245 Explanation: just did it on edge 2021 Correct 👌 arrow right Explore similar answers messages

Source Image: instructables.com
Download Image
Steps for Problem Solving How many moles of oxygen react with hydrogen to produce 27.6 mol of \(\ceH2O\)? Find a balanced equation that describes the reaction. Unbalanced: H 2 + O 2 → H 2 O. Balanced: 2H 2 + O 2 → 2H 2 O. Identify the “given” information and what the problem is asking you to “find.” Given: moles H 2 O Find: moles oxygen
Source Image: slideplayer.com
Download Image
Stoichiometry And Limiting Reagent Review – Quiz, Trivia & Questions Study with Quizlet and memorize flashcards containing terms like 1. A student tried to solve the following problem by selecting the tile as shown. What, if anything, did the student do wrong?, 2. Based on the tiles, shown, how many moles of oxygen gas (O2) are needed to produce 36.04 grams of water (H2O) when reacting with access ethane in the equation: 2C2H6 + 7O2 —> 5CO2 + 6H2O?, 3.
(491).jpg)
Source Image: proprofs.com
Download Image
Solve The Problem For The Moles Of Oxygen. Mol O2
Study with Quizlet and memorize flashcards containing terms like 1. A student tried to solve the following problem by selecting the tile as shown. What, if anything, did the student do wrong?, 2. Based on the tiles, shown, how many moles of oxygen gas (O2) are needed to produce 36.04 grams of water (H2O) when reacting with access ethane in the equation: 2C2H6 + 7O2 —> 5CO2 + 6H2O?, 3. A bit confusing? I will elaborate on this below. After setting up the proportion, you will cross-multiply and divide to get the answer. Here is the first equation we’ll use: Example #1: When 2.00 mol of N reacts with sufficient H, how many moles of NH will be produced? Comments prior to solving the example (a) The equation is already balanced.
MCQs On Stoichiometry And Stoichiometric Calculations – Quiz, Trivia & Questions
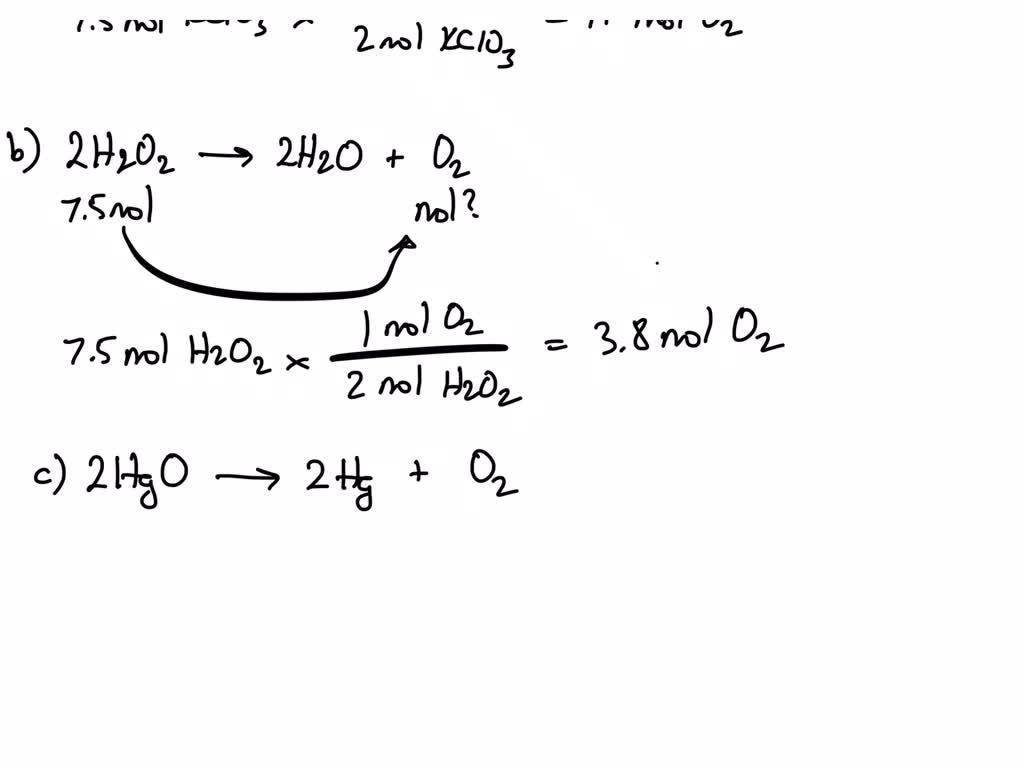
Step 1: Convert known reactant mass to moles. In order to relate the amounts H A 2 SO A 4 and NaOH using a mole ratio, we first need to know the quantity of H A 2 SO A 4 in moles. We can convert the 3.10 grams of H A 2 SO A 4 to moles using the molar mass of H A 2 SO A 4 ( 98.08 g / mol ): 8.1 Reacts with 3 mol of O2 Produces 1 mol of Al2O3 – ppt video online download

Source Image: slideplayer.com
Download Image
Calculate the mass of 5 moles of Oxygen and also calculate the number of molecules present in it also calculate – Chemistry – Some Basic Concepts of Chemistry – 12928067 | Meritnation.com Step 1: Convert known reactant mass to moles. In order to relate the amounts H A 2 SO A 4 and NaOH using a mole ratio, we first need to know the quantity of H A 2 SO A 4 in moles. We can convert the 3.10 grams of H A 2 SO A 4 to moles using the molar mass of H A 2 SO A 4 ( 98.08 g / mol ):

Source Image: meritnation.com
Download Image
Converting Mass of Reactant to Mass of Product — Examples – Expii Step 1. Write the balanced equation. 2KClO3 → 2KCl+ 3O2 Step 2. Calculate the moles of KClO3. 10.0g KClO₃ × 1 mol KClO3 122.6g KClO₃ = 0.08156 mol KClO3 Step 3. Calculate the moles of O2. The balanced equation tells us that 2 mol KClO3 give 3 mol O2. Therefore, 0.08156mol KClO₃ × 3 mol O2 2mol KClO₃ = 0.1223 mol O2 Step 4.

Source Image: expii.com
Download Image
Stoichiometry And Limiting Reagent Review – Quiz, Trivia & Questions Steps for Problem Solving How many moles of oxygen react with hydrogen to produce 27.6 mol of \(\ceH2O\)? Find a balanced equation that describes the reaction. Unbalanced: H 2 + O 2 → H 2 O. Balanced: 2H 2 + O 2 → 2H 2 O. Identify the “given” information and what the problem is asking you to “find.” Given: moles H 2 O Find: moles oxygen
Source Image: proprofs.com
Download Image
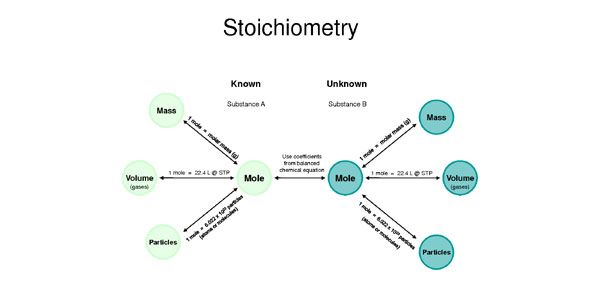
Solved Determine the moles of oxygen gas (O2) needed to | Chegg.com Conversions Between Moles and Gas Volume. Molar volume at STP can be used to convert from moles to gas volume and from gas volume to moles. The equality of 1mol = 22.4L 1 mol = 22.4 L is the basis for the conversion factor. Many metals react with acids to produce hydrogen gas. A certain reaction produces 86.5 L 86.5 L of hydrogen gas at STP.
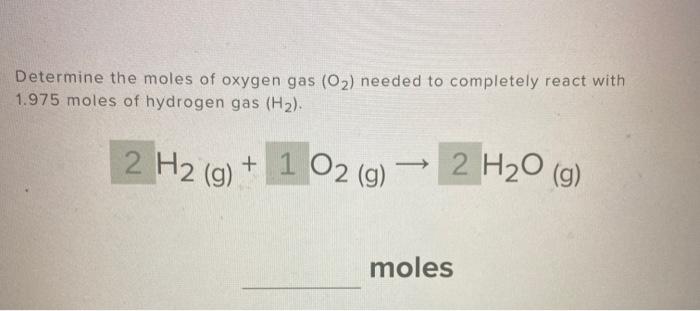
Source Image: chegg.com
Download Image
⏩SOLVED:How many moles of oxygen can be obtained by the… | Numerade Study with Quizlet and memorize flashcards containing terms like 1. A student tried to solve the following problem by selecting the tile as shown. What, if anything, did the student do wrong?, 2. Based on the tiles, shown, how many moles of oxygen gas (O2) are needed to produce 36.04 grams of water (H2O) when reacting with access ethane in the equation: 2C2H6 + 7O2 —> 5CO2 + 6H2O?, 3.
Source Image: numerade.com
Download Image
7.57 How many moles of oxygen needed to react with 4 moles hydrogen? – YouTube A bit confusing? I will elaborate on this below. After setting up the proportion, you will cross-multiply and divide to get the answer. Here is the first equation we’ll use: Example #1: When 2.00 mol of N reacts with sufficient H, how many moles of NH will be produced? Comments prior to solving the example (a) The equation is already balanced.

Source Image: m.youtube.com
Download Image
Calculate the mass of 5 moles of Oxygen and also calculate the number of molecules present in it also calculate – Chemistry – Some Basic Concepts of Chemistry – 12928067 | Meritnation.com
7.57 How many moles of oxygen needed to react with 4 moles hydrogen? – YouTube 1 Answer: 0.245 Explanation: just did it on edge 2021 Correct 👌 arrow right Explore similar answers messages
Stoichiometry And Limiting Reagent Review – Quiz, Trivia & Questions ⏩SOLVED:How many moles of oxygen can be obtained by the… | Numerade Conversions Between Moles and Gas Volume. Molar volume at STP can be used to convert from moles to gas volume and from gas volume to moles. The equality of 1mol = 22.4L 1 mol = 22.4 L is the basis for the conversion factor. Many metals react with acids to produce hydrogen gas. A certain reaction produces 86.5 L 86.5 L of hydrogen gas at STP.